
- #Atomic emission spectrum scarf pdf
- #Atomic emission spectrum scarf series
- #Atomic emission spectrum scarf download
Elements can be identified by their emission spectra. Each element has its own unique line spectra (fingerprint).
#Atomic emission spectrum scarf series
#Atomic emission spectrum scarf pdf
pdf and submit the worksheet on time and to your TA in the dropbox on D2L.\right)\). Once the worksheet is complete save as a.
#Atomic emission spectrum scarf download
Complete the above pages using the Microsoft version of this file that is available for download on the lab D2L page. There is not a formal lab report for this lab.

The y-axis and on the x-axis, the resulting slope will be the RydbergĬonstant, R H.

If the wavelength is short, there will be a largerįrequency and as consequence, more energy.Ĭompare the energy values from Table 1. This is because Indonesian currency coin is m ade of nickel and iron elements. Furthermore, Neut ral iron line (Fe) at 507.405 also occurs clearly as shown in the figure. Nickel emission lines strongl y appear at the same wavelength as in the case of nickel metal plate. well defined signatures in the absorption spectrum. The amount of energy depends on the length of the emission spectrum obtained from the Indonesian currencycoin. 3.7 A rigorous quantum-mechanical treatment of radiation induced atomic dynamics in. Wavelength and energy have a corresponding relationship along What is the relationship between wavelength and energy? Planck’s constant and c is the speed of light. The formula for the determination of the energy of light is E = where h is Just describe the peak by its wavelength.Ĭonvert the wavelength to units of meters and enter in Table 1. Even if youĪre unable to visibly see the fourth peak, the spectrometer can detect it, you can (Round wavelength values to whole numbers).
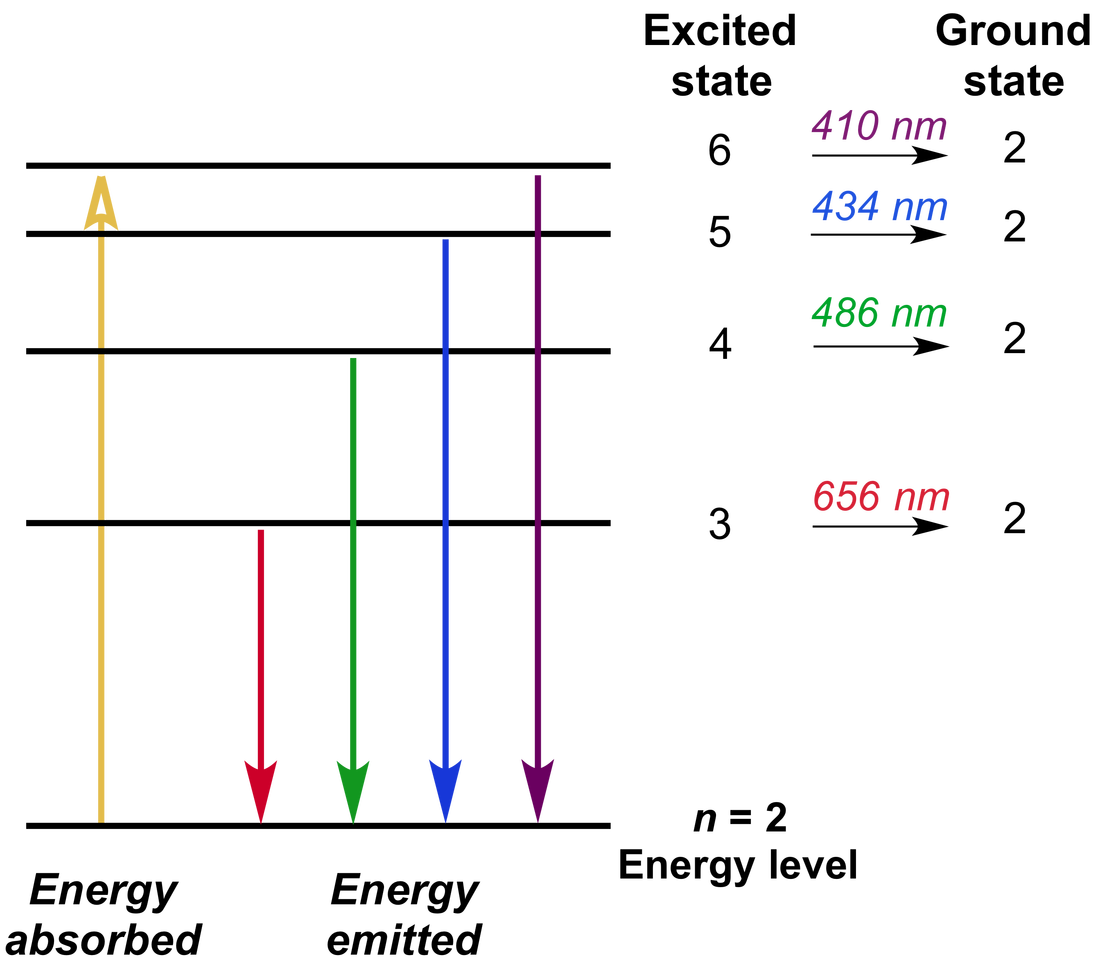
Record the colors and wavelengths of the four peaks in the visible hydrogen You can reset the zoom feature by toggling the visible/full You can also use the cursor to select a region of the It will identify the wavelength (in nm) in the x-coordinate field in the bottom rightĬorner of the detector window. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Three of them if your eyes are like the authors. You will seeįour peaks in the spectrum, but may only be able to see the corresponding colors of Peaks are shown in the window above the spectra.Ĭlick on the Visible/Full switch to magnify only the visible spectrum. The hydrogen emission spectra is displayed in the window in the upper right corner The center of the table has a gas discharge tube containing hydrogen gas. The Spectrometer will be on the right of the lab Then select ‘The Rydberg Equation’ from the list of assignments. The Rydberg Equation an Atomic Emission Spectra Activity Part A, Determination of the Rydberg Constantįrom the Beyond Labz portal, start Virtual Chem Lab, select Atomic Theory, and


 0 kommentar(er)
0 kommentar(er)
